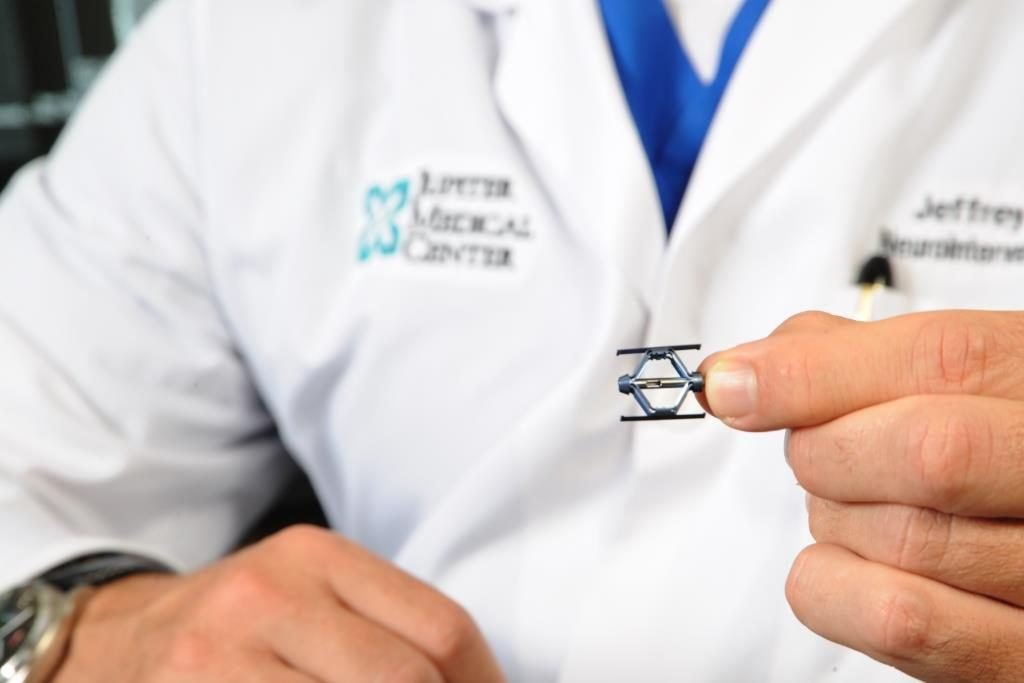
Jupiter, Florida, Dec. 05, 2018 (GLOBE NEWSWIRE) — JUPITER, FLORIDA (December 5, 2018) — Jupiter Medical Center is the first hospital in the southeastern United States to use SpineJack®, an implantable system that relieves the pain of acute vertebral compression fractures (VCFs), the most common fracture in patients with osteoporosis. The innovative device stabilizes and restores damaged vertebra to their original shape and height in a minimally invasive procedure that takes about 30 minutes.
“We take pride in offering the most advanced treatment options available,” said Don McKenna, president and chief executive officer of Jupiter Medical Center. “With this new technology, we are establishing Jupiter Medical Center as a leader in spine care not just locally, but nationally.”
Approved by the U.S. Food and Drug Administration in September, SpineJack® has been commercially available in Europe since 2008, with more than 70,000 units implanted worldwide. It is produced domestically by the Stryker medical technology company.
“This is a revolutionary advancement for treating many patients suffering from acute osteoporotic vertebral compression fractures,” said Dr. Jeffrey Miller, medical director of Neuroendovascular Surgery and co-medical director of the Stroke Program at Jupiter Medical Center. “We know from its success in Europe over the last decade that it significantly improves the patient’s quality of life almost immediately.”
“SpineJack® is part of the expanding array of treatment options available in Jupiter Medical Center’s Interventional Radiology Program,” said Dr. Lee A. Fox, medical director of Imaging at Jupiter Medical Center. “Our program offers a full scope of advanced diagnostic procedures and minimally invasive therapies, and we are delighted to bring this new treatment option to our patients.”
 Dr. Miller performed Jupiter Medical Center’s first SpineJack® on Nov. 6. His patient, 75-year-old Robert Fischer of North Palm Beach, is pleased with the outcome. “It’s only been three weeks and I feel close to perfect – I’d say 95 percent,” said Fischer, who was diagnosed with osteoporosis earlier this year after he began suffering debilitating back pain.
Dr. Miller performed Jupiter Medical Center’s first SpineJack® on Nov. 6. His patient, 75-year-old Robert Fischer of North Palm Beach, is pleased with the outcome. “It’s only been three weeks and I feel close to perfect – I’d say 95 percent,” said Fischer, who was diagnosed with osteoporosis earlier this year after he began suffering debilitating back pain.
VCFs are the most common medical condition experienced by those with osteoporosis or low bone mass. VCFs occur when the vertebral body in the spine collapses, which can lead to severe pain, spinal deformity and loss of height. The fractures more commonly occur in the middle (thoracic) and lower (lumbar) portions of the spine. While osteoporosis is the most common cause, these fractures may also be caused by trauma or metastatic tumors. The lifetime risk of osteoporotic fracture for those older than 50 years of age is one in two for women and one in four for men.
“SpineJack® treats back pain by targeting the cause and, as a result, it reduces the need for long-term use of pain medication,” Dr. Miller said. “In addition to enhancing our patients’ quality of life, this procedure could have a significant impact in the effort to combat opioid addiction.”
For decades the only nonsurgical treatments for VCFs were bed rest, bracing (which limits mobility) and pain medication. Vertebroplasty has been used since the early 1990s and more recently kyphoplasty, which has become a mainstay in the treatment of VCFs. In vertebroplasty, bone cement is injected directly into the fractured vertebra, where it quickly hardens and acts like an internal cast. In kyphoplasty, a balloon is inserted into the fracture to expand the space before the cement is added. Neither procedure restores the vertebral body to its full height.
SpineJack® was invented by a French physician whose inspiration was a scissor jack, the diamond-shaped car jack that is commonly used to lift a vehicle when changing a flat tire. Instead of a kyphoplasty balloon, the procedure utilizes a titanium implant that resembles a tiny scissor jack, which is deployed into the fractured vertebral body. Once in place, the SpineJack® is expanded to lift the compressed vertebra and restore it to its normal height. Two implants are used in the procedure, one on each side of the vertebral body. They are locked into the desired expanded position and bone cement is then injected to stabilize the vertebra. The implant becomes encapsulated with bone cement resulting in pain relief for the patient.
Fischer proved to be the ideal patient to undergo the first SpineJack® at Jupiter Medical Center. In August, while spending the summer in Rhode Island, he underwent a kyphoplasty procedure to treat a VCF in his upper back. While wintering in South Florida just a few months later, he suffered a second VCF during a round of golf—this time in his lower back. His primary care physician referred him to Dr. Miller, who thought he would be a good candidate for SpineJack®.
“My recovery was much quicker,” he said. “With (kyphoplasty), that recovery took about 10 weeks before I felt as good as SpineJack® feels now, just three weeks later. I would definitely recommend it.”
Performed on an outpatient basis, the procedure is appropriate for patients who have been diagnosed with a VCF within four to six months of treatment. For more information, visit https://www.jupitermed.com/spinejack or call 561-263-2200.
About Jupiter Medical Center
A not-for-profit 327-bed regional medical center consisting of 207 private acute-care hospital beds and 120 long-term care, sub-acute rehabilitation and Hospice beds, Jupiter Medical Center is reimagining how to restore the community’s health and wellness. Award-winning physicians, world-class partnerships and innovative techniques and technology enable Jupiter Medical Center to provide a broad range of services with specialty concentrations in cardiology, oncology, imaging, orthopedics & spine, digestive health, emergency and pediatric services, lung & thoracic, women’s health, weight management and men’s health.
Founded in 1979, Jupiter Medical Center has approximately 1,650 team members, 637 physicians and 640 volunteers. Jupiter Medical Center continues to perform in the top 10 percent of hospitals for patient quality and satisfaction. For more information on Jupiter Medical Center, please call (561) 263-2234 or visit www.jupitermed.com.
Ernestine Williams
Jupiter Medical Center
5612633852
Ernestine.Williams@jupitermed.com